Global Warming Science - www.appinsys.com/GlobalWarming
Ocean Acidification
[last update: 2010/06/20]
|
Ocean acidification has become the next scare since CO2 isn’t producing significant warming. The BBC refers to ocean acidification as “the other CO2 problem” in their article “Acid Oceans Need Urgent Action” [http://news.bbc.co.uk/2/hi/science/nature/7860350.stm]: “The world's marine ecosystems risk being severely damaged by ocean acidification unless there are dramatic cuts in CO2 emissions, warn scientists.” Referring to the “Monaco Declaration”, “It says pH levels are changing 100 times faster than natural variability. We scientists who met in Monaco to review what is known about ocean acidification declare that we are deeply concerned by recent, rapid changes in ocean chemistry and their potential, within decades, to severely affect marine organisms, food webs, biodiversity and fisheries. … could make most regions of the ocean inhospitable to coral reefs by 2050, if atmospheric CO2 levels continue to increase. … changes so rapid and severe that impacts on organisms appear unavoidable. The questions are now how bad will it be and how soon will it happen.”
The above sounds scary, but the scientific problem with the alarmist scare stories is a lack of historical data and a lack of data on the actual biological effects. “A lack of baseline information on the pH of coastal waters is one stumbling block. … only three sites currently have data on ocean pH that span a sufficient amount of time to be useful to regulators. One is in Monterey Bay off California. The other two are open-ocean sites. … Moreover, a dearth of information on the biological effects of increased acidity still exists” [http://www.csmonitor.com/USA/2010/0312/Ocean-acidification-another-path-to-EPA-rules-on-carbon-emissions] (Monterey Bay data show decreased acidity as discussed below.)
|
|
Definition: Acidic: containing or having the properties of an acid, “Pertaining to substances that have a ph lower than 7” [http://www.biology-online.org/dictionary/Acidic]. While the term acidification refers to decreasing pH, the term acidic is only valid when the pH is less than 7.
The concern is that as the atmospheric CO2 increases, it increases the CO2 content of the ocean, resulting in increased carbonic acid (H2CO3). The following equation shows the basic equilibrium equation involved in the water (with <=> denoting equilibrium):
CO2 + H2O <=> H2CO3 <=> H+ + HCO3-
From Wikipedia [http://en.wikipedia.org/wiki/Carbonic_acid]: “The hydration equilibrium constant at 25°C is Kh= 1.70×10−3: hence, the majority of the carbon dioxide is not converted into carbonic acid and stays as CO2 molecules. In the absence of a catalyst, the equilibrium is reached quite slowly.” This is also temperature dependent – the dissociation constant increases with temperature resulting in more H+ at higher temperatures.
Of course sea water is not just H2O with dissolved CO2 and thus the simple equation above ignores all the other chemicals that are actually involved with the reactions in the ocean, but it provides the basis of the concern. Due to the complexity of the chemistry of the oceans and the biological processes involved, only a spatially large set of data over a long term can provide any real basis for knowledge in this area. This is a major problem, since consistent pH measurement data sets have only recently been made at very few locations and for very few years (“100 times faster than natural variability” – if only that natural variability were known). The problem is a lack of historical data.
The biological concern is based on the equilibrium equation for calcium carbonate used by animals to create shells etc.
CaCO3 <=> Ca + CO3-
Oceanic sediments have abundant CaCO3 and this buffers the acidification.
The Royal Society published a report “Ocean acidification due to increasing atmospheric carbon dioxide” Royal Society Policy document 12/05, June 2005 [http://royalsociety.org/displaypagedoc.asp?id=13314]. It stated: “Calculations based on measurements of the surface oceans and our knowledge of ocean chemistry indicate that this uptake of CO2 has led to a reduction of the pH of surface seawater of 0.1 units. If global emissions of CO2 from human activities continue to rise on current trends then the average pH of the oceans could fall by 0.5 units (equivalent to a threefold increase in the concentration of hydrogen ions) by the year 2100. This pH is probably lower than has been experienced for hundreds of millennia and, critically, this rate of change is probably one hundred times greater than at any time over this period.“ Note that this is based on calculations – not measurements of pH. [Note also, that the Royal Society, whose motto coined in 1663 contained the phrase “on the word of no one”, has dropped that phrase from their web site and now wants everyone to “respect the facts” http://www.spiked-online.com/index.php?/site/article/3357/]
The report further states: “Research into the impacts of high concentrations of CO2 in the oceans is in its infancy. …Predicting the direction and magnitude of changes in a complex and poorly studied system such as the oceans is very difficult. However, there is convincing evidence to suggest that acidification will affect the process of calcification, by which animals such as corals and molluscs make shells and plates from calcium carbonate.”
The RS report states: “The tropical and subtropical corals are expected to be among the worst affected… Other calcifying organisms that may be affected are components of the phytoplankton and the zooplankton, and are a major food source for fish and other animals. Regional variations in pH will mean that by 2100 the process of calcification may have become extremely difficult for these groups of organisms.”
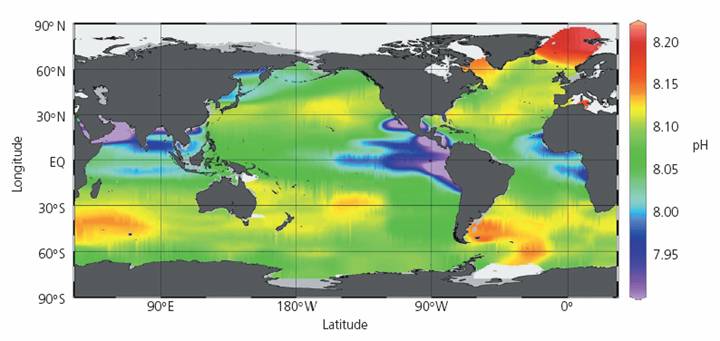
The following figure is from the Royal Society report cited above. It shows a “Map of mixed surface layer (upper 50 m) pH values in the global oceans for the nominal year 1994. The lowest values are observed in upwelling regions (eg Equatorial Pacific, Arabian Sea) where subsurface waters with lower pH values are brought to the surface. The highest values are observed in regions of high biological production and export. In these regions DIC is fixed by phytoplankton and exported by the biological pump into the deeper layers resulting in higher pH values in the surface waters.” If the lowest values are observed where upwelling surface waters are brought to the surface, it indicates that it is not caused by atmospheric CO2, which would have greater effects near the surface. In fact the report states: “In the deep oceans, the CO2 concentration increases as sinking organic matter from biological production (which varies seasonally) is decomposed. These additions of CO2 to the deep oceans cause its pH to decrease … When this CO2-rich deep water upwells to the surface, it creates regions with lower pH in the surface waters”. No atmospheric CO2 required for that.
The same report states: “Carbon dioxide in the atmosphere dissolves in the surface waters of the oceans and establishes a concentration in equilibrium with that of the atmosphere. Molecules of CO2 exchange readily with the atmosphere and on average only remain in the surface waters for about 6 years. … Surface oceans have an average pH globally of about 8.2 units. However, pH can vary by ±0.3 units due to local, regional and seasonal factors.” The stated 8.2 ±0.3 number does not match the figure they present shown above.
A 2006 paper (Loaiciga “Modern-age buildup of CO2 and its effects on seawater acidity and salinity”, Geophysical Research Letters, Vol.33, 2006 [http://www.agu.org/pubs/crossref/2006.../2006GL026305.shtml]) concludes: “results concerning average seawater salinity and acidity show that, on a global scale and over the time scales considered (hundreds of years), there would not be accentuated changes in either seawater salinity or acidity from the observed or hypothesized rises in atmospheric CO2 concentrations”
|
|
Ocean Acidification Studies
A 2009 Study (Dore et al, “Physical and biogeochemical modulation of ocean acidification in the central North Pacific”, Proc. Natl Acad Sci, July 2009) states: “Although the physical and chemical basis for ocean acidification is well understood, there exist few field data of sufficient duration, resolution, and accuracy to document the acidification rate and to elucidate the factors governing its variability. … We suggest that physical and biogeochemical processes alter the acidification rate with depth and time and must therefore be given due consideration when designing and interpreting ocean pH monitoring efforts and predictive models.” [http://www.ncbi.nlm.nih.gov/pubmed/19666624]
Washington State
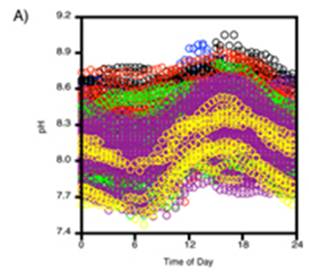
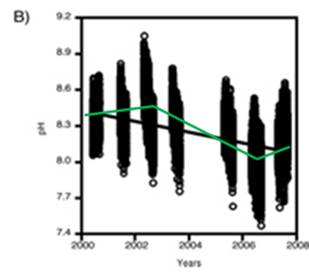
A study of pH since 2000 at one location off the northwestern tip of Washington State [http://pondside.uchicago.edu/ecol-evol/faculty/Wootton/pH.htm] states: “Since 2000, we have been monitoring physical ocean conditions, including ocean pH, at our main study site in the northeastern Pacific Ocean: Tatoosh Island, Washington, USA. … Even over the course of a day, pH typically varies by 0.24 units, a consequence of the uptake and production of CO2 through photosynthesis and respiration. Hence biological processes, which are often left out of models of ocean pH, can have strong effects. … Over 70% of the variability in pH we observed can be related to changes in a small set of factors with known mechanistic links to pH: atmospheric CO2, water temperature, the daily photosynthesis-respiration cycle, phytoplankton abundance, upwelling of high CO2 subsurface water, alkalinity, salinity, and the Pacific Decadal Oscillation.” Note that 70% of the variability is not attributed to atmospheric CO2, but includes phytoplankton abundance, water temperature and upwelling of subsurface water.
The following figure is from the above study. It shows: A) daily variation in pH, and B) the pH measurements over the years in the study. An alarmist press release for this research stated: “The acidity increased more than 10 times faster than had been predicted by climate change models and other studies” – but they have data observed over a period of only 8 years!
Hawaii
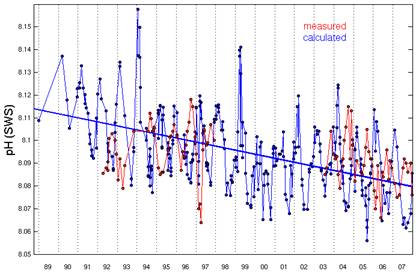
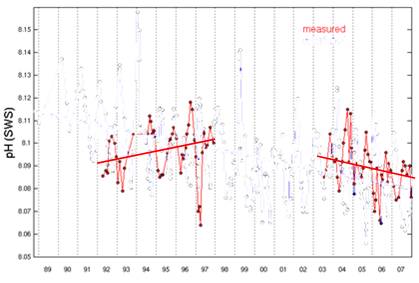
The Hawaii Ocean Time-series began pH measurements in Hawaii in 1992. [http://hahana.soest.hawaii.edu/hot/trends/trends.html] The following graph is from the graphing page on that web site. The web site does not explain the difference between the “measured” and “calculated” data, but apparently the “calculated” uses other non-pH measurements to calculate pH.
The following figure is the same as above with the “calculated” removed.
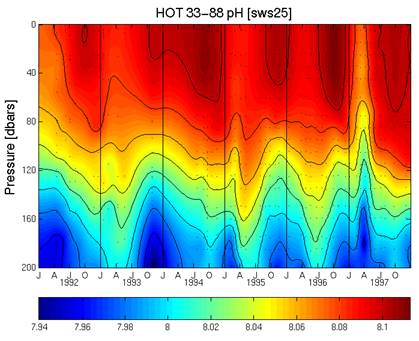
The following figure is from the same web site [http://hahana.soest.hawaii.edu/hot/methods/fig19.gif] and shows the pH profile with depth from 1992 to 1997. It can be seen here that the pH was increasing from 1992 to 1997, which matches the “measured” above – not the “calculated”.
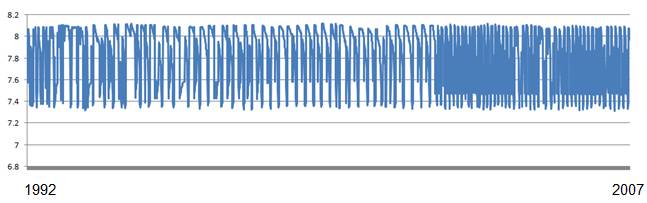
The above web site also provides data extraction. The following figure shows the data graphed in Excel (1992 to 2007). The point evident from the data is that annual pH variation greatly exceeds any change over time in this 15-year data set.
Hawaii to Alaska
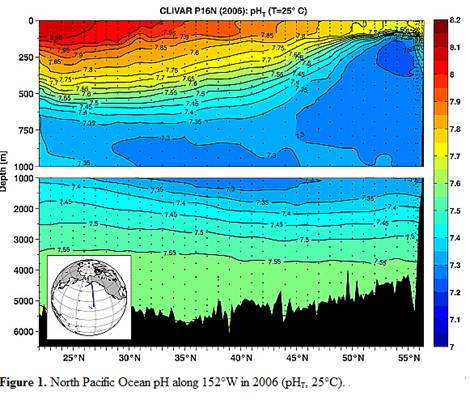
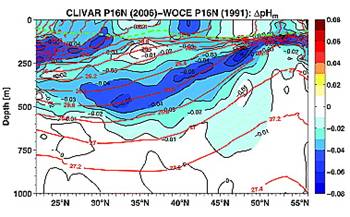
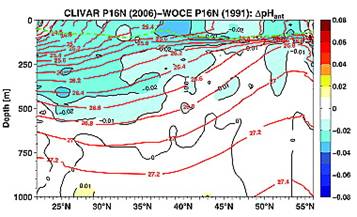
A study reported in 2010 (Byrne et al: “Direct observations of basin-wide acidification of the North Pacific Ocean”, Geophysical Research Letters Vol. 37, 2010 [http://www.agu.org/journals/gl/gl1002/2009GL040999/]) measured ocean pH along a transect from near Hawaii to Alaska over a period of 15 years. The following Figure 1 is from their paper showing the 2006 pH along the transect.
Notice above that pH changes from 8.1 to 7.6 at the surface just by traveling from Hawaii to Alaska. The changes are even greater at 250m depth.
The following figures from their paper show the change in pH between 1991 and 2006 (left) and the change in pH attributed to anthropogenic causes (right – although no scientific justification was given in the paper for deciding on the amount of anthropogenic contribution).
The amount attributed to anthropogenic cause over 15 years is smaller than the daily variation in Hawaii.
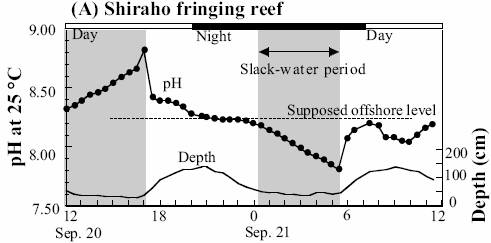
Shiraho Reef, Western Pacific
The following figure shows the daily variation in pH for two days at Shiraho reef [http://www.terrapub.co.jp/e-library/kawahata/pdf/229.pdf]
When the daily pH variation (as shown above for Shiraho and previously for Hawaii) is much greater than the slight change detected over 15 years, and since the background variation studies are still very short-term, any attribution to anthropogenic CO2 is not supported by data.
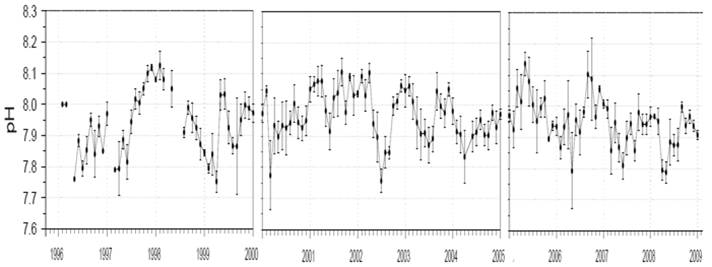
The following figure shows the monthly mean pH measured in the incoming water to the Monterey Bay Aquarium drawn from a ~50ft depth for 1996 to 2009. As can be seen pH varies constantly and shows no trend towards acidification. [http://sanctuarymonitoring.org/regional_docs/monitoring_projects/100240_167.pdf]
|
|
Phytoplankton
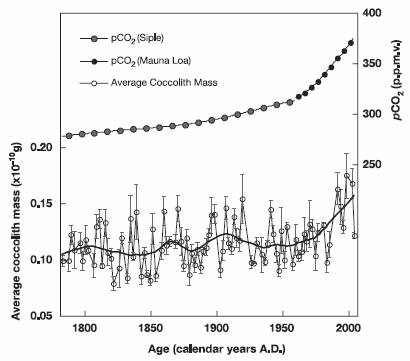
A study of phytoplankton calcification in the Atlantic (“Phytoplankton Calcification in a High-CO2 World” [http://www.sb-roscoff.fr/Phyto/index.php?option=com_docman&task=doc_details&gid=418&Itemid=112]) states: “From the mid-Mesozoic, coccolithophores have been major calcium carbonate producers in the world’s oceans, today accounting for about a third of the total marine CaCO3 production. Here, we present laboratory evidence that calcification and net primary production in the coccolithophore species Emiliania huxleyi are significantly increased by high CO2 partial pressures. Field evidence from the deep ocean is consistent with these laboratory conclusions, indicating that over the past 220 years there has been a 40% increase in average coccolith mass.”
The following figure is from that study.
An experimental study of coccolithopores and the effects of increasing CO2 showed the following [http://oceanacidification.wordpress.com/2008/04/24/can-seashells-save-the-world/]: “Debora Iglesias-Rodriguez, a biological oceanographer at the National Oceanographic Centre in Southampton, carried out the experiments by bubbling carbon dioxide into tanks to see how the species would cope with rising levels of the dissolved gas. She found that the single-celled plant actually excreted bigger plates at higher concentrations. In fact, at levels of carbon dioxide in the experiment reaching 750ppm (about double of those today), the calcification rates also doubled compared with the calcification rates of coccolithophores grown at CO2 levels of 280ppm, which is to say, pre-industrial levels. … “Our widely held assumption that the acidification of the oceans causes a decrease in calcification in all coccolithophores needs to be reappraised,” says Dr Iglesias-Rodriguez. “Our data reveal that these microscopic organisms have been responding to climate change by increasing the size of the cells and their calcium carbonate plates.” … Previous experiments with coccolithophores suggested that as acidity levels increased, calcification would decrease. However, Dr Iglesias-Rodriguez believes this may have been due to the way the experiments were carried out. The scientists simply added acid to the water to mimic the increase in acidity due to dissolved carbon dioxide. Her method was to simulate the more natural process by bubbling the gas through the water until it dissolved. “This work contradicts previous findings and shows, for the first time, that calcification by phytoplankton could double by the end of this century,” she says. “This is important because the majority of ocean calcification is carried out by coccolithophores such as Emiliania huxleyi and the amount of calcium carbonate produced at the ocean surface is known to have a direct influence on levels of atmospheric carbon dioxide.”
|
|
Coral
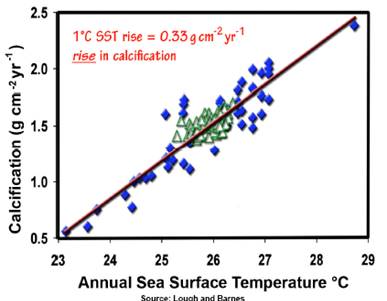
A study of calcification of corals on the Australian Great Barrier Reef (Lough, J.B. and Barnes, D.J. 2000. “Environmental controls on growth of the massive coral Porites”, Journal of Experimental Marine Biology and Ecology 245: 225-243) states: “The response of calcification rate to temperature remained linear. Variation in annual average SST accounted for 84% of the variance. For each 1°C rise in SST, average annual calcification increased by 0.33 g cm−2 year−1 and average annual extension increased by 3.1 mm year−1 (c.f. average values of 1.50 g cm−2 year−1 and 11.6 mm year−1, respectively). The sensitivity of calcification rate in Porites to SST, combined with observed 20th Century increases in SSTs, suggests that calcification rates may have already significantly increased along the GBR in response to global climate change.” Contrary to the doomsday models, empirical evidence shows an increase in calcification. [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T8F-3YN92RJ-6&_user=10&_rdoc=1&_fmt=&_orig=search &_sort=d&view=c&_acct=C000050221&_version=1 &_urlVersion=0&_userid=10&md5=112bc544f861b4740fd0662e28ca0a68]
The following figure is from the data in the above study (from http://scienceandpublicpolicy.org/images/stories/papers/originals/coral_co2_warming.pdf - this PDF describes many of the studies on pH effects on various calcifying sea creatures. After reviewing the various studies, this PDF concludes: “If there is a lesson to be learned from the materials discussed in this review, it is that people should be paying much more attention to real-world observations than to theoretical predictions. Far too many predictions of CO2-induced catastrophes are treated as sure-to-occur, when real-world observations show them to be highly unlikely or even virtual impossibilities. The cases of CO2-induced coral bleaching and ocean acidification are no different.”)
A 2009 study (Rodolfo-Metalpa et al, “Response of the temperate coral Cladocora caespitosa to mid- and long-term exposure to pCO2 and temperature levels projected in 2100”) states: “While temperature was found as one of the most critical environmental parameter controlling the physiology and calcification of C. caespitosa, an increase in CO2 concentration, within the values expected to the end of 2100, did not affect significantly either their photosynthetic performance or the calcification rates.” [http://www.biogeosciences-discuss.net/6/7103/2009/bgd-6-7103-2009.pdf]
Biology can influence calcium carbonate precipitation significantly. The ocean acidification scare is based on bulk solution chemistry, but biological surfaces have big influences at on local solution chemistry at the molecular level. The buffering of CO2-produced carbonic acid is something that living organisms deal with. The following description is from Wikipedia [http://en.wikipedia.org/wiki/Carbonic_acid]: “Carbonic acid is an intermediate step in the transport of CO2 out of the body via respiratory gas exchange. The hydration reaction of CO2 is generally very slow in the absence of a catalyst, but red blood cells contain carbonic anhydrase which both increases the reaction rate and disassociates a hydrogen ion (H+) from the resulting carbonic acid, leaving bicarbonate (HCO3-) dissolved in the blood plasma. This catalysed reaction is reversed in the lungs, where it converts the bicarbonate back into CO2 and allows it to be expelled. Carbonic acid also plays a very important role as a buffer in mammalian blood. The equilibrium between carbon dioxide and carbonic acid is very important for controlling the acidity of body fluids, and the carbonic anhydrase increases the reaction rate by a factor of nearly a billion to keep the fluids at a stable pH.”
Corals cope with acidification in a similar manner to humans, as described in the research paper published in the Journal of Biological Chemistry: “Carbonic Anhydrase in the Scleractinian Coral” at this link: [http://www.jbc.org/cgi/content/abstract/283/37/25475]
December 2008: 4 Years After Tsunami Corals Stage Comeback [http://www.physorg.com/news149768973.html]: “A team of scientists from the New York-based Wildlife Conservation Society (WCS) has reported a rapid recovery of coral reefs in areas of Indonesia, following the tsunami that devastated coastal regions throughout the Indian Ocean four years ago today. The team, which has surveyed the region's coral reefs since the December 26, 2004 tsunami, looked at 60 sites along 800 kilometers (497 miles) of coastline in Aceh, Indonesia. The researchers attribute the recovery to natural colonization by resilient coral species, along with the reduction of destructive fishing practices by local communities. While initial surveys immediately following the tsunami showed patchy (albeit devastating) damage to coral reefs in the region, surveys in 2005 indicated that many of the dead reefs in the study area had actually succumbed long ago to destructive fishing practices such as the use of dynamite and cyanide to catch fish. It is also possible that the crown of thorns starfish—a marine predator—had caused widespread coral mortality.”
|
|
Crabs and Shrimp
Woods Hole Oceanographic Institution http://www.whoi.edu/page.do?pid=7545&tid=282&cid=63809&ct=162
|
|
As the alarmists say: “changes so rapid and severe that impacts on organisms appear unavoidable. The questions are now how bad will it be and how soon will it happen.” The problem as usual is a lack of empirical evidence – just alarm about the future.
See also: Floor Anthoni at Sea Friends (http://www.seafriends.org.nz/issues/global/acid2.htm) provides an extensive examination of the alarmist ocean acidification articles.
|
|
|